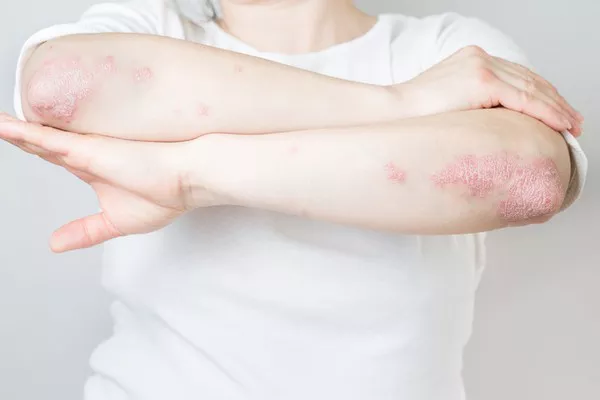
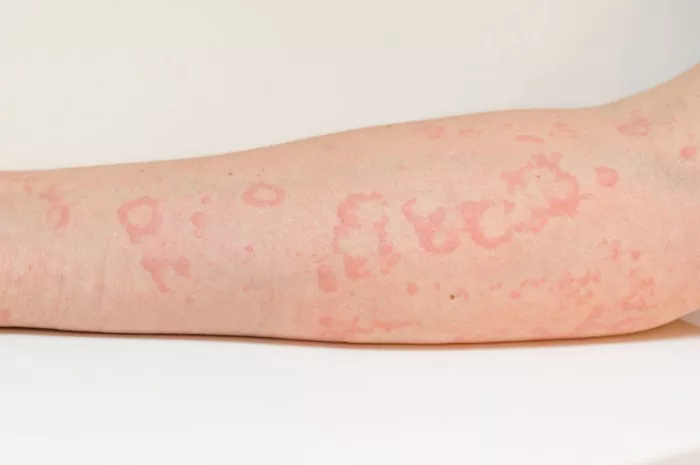
A recent study spearheaded by Matteo Megna from the University of Naples Federico II, Italy, has bolstered the efficacy and safety claims surrounding guselkumab as a viable treatment for plaque psoriasis, particularly following prolonged ustekinumab use and subsequent discontinuation. While promising, researchers caution the need for further validation through additional studies.
The investigation aimed to evaluate the effectiveness and safety profile of guselkumab in psoriasis patients who exhibited inadequate responses to ustekinumab over a span of up to 3 years. Although prior trials such as NAVIGATE had explored guselkumab’s utility in moderate-to-severe psoriasis cases with suboptimal ustekinumab responses, real-world confirmation was deemed imperative by the investigators.
“Real-world data are highly needed to confirm trial data in a more complicated setting and also because guselkumab and ustekinumab partially share their therapeutic target…,” noted Megna and colleagues.
Methodology:
The multicenter retrospective trial involved assessing patients with moderate-to-severe plaque psoriasis across nine centers in Italy’s Campania region. Demographic information, comorbidities, and clinical characteristics were scrutinized, along with data on prior and current systemic psoriasis medications, focusing on ustekinumab usage and reasons for discontinuation.
Researchers monitored Psoriasis Activity Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores at baseline and subsequent follow-ups at 16, 36, 52, 104, and 156 weeks. Treatment-emergent adverse events (AEs) were recorded through physical evaluations and laboratory tests.
Findings:
The study comprised 112 participants, predominantly male with an average age of 54.8 years. Noteworthy improvements were observed as early as the sixteenth week, with sustained efficacy recorded throughout the 156-week period. Importantly, no serious AEs were reported during the study duration.
Subgroup analysis revealed that patients with psoriatic arthritis or palmoplantar psoriasis exhibited lesser clinical improvement at 16 and 36 weeks, though no significant differences were observed by Week 52. Nail involvement, body mass index (BMI), and affected regions like the genital or scalp areas did not appear to impact medication effectiveness.
The researchers underscored the significance of their 156-week real-world study, asserting that switching from ustekinumab to guselkumab did not compromise the latter’s effectiveness in targeting the anti-IL 23 pathway. Limitations included the study’s retrospective nature, relatively small cohort size, and limited subjects with extended follow-up periods, potentially affecting generalizability.
In summary, the findings offer substantial support for guselkumab as a safe and effective alternative for psoriasis patients with inadequate responses to ustekinumab, warranting further investigation to solidify its clinical standing.
Related Topics: