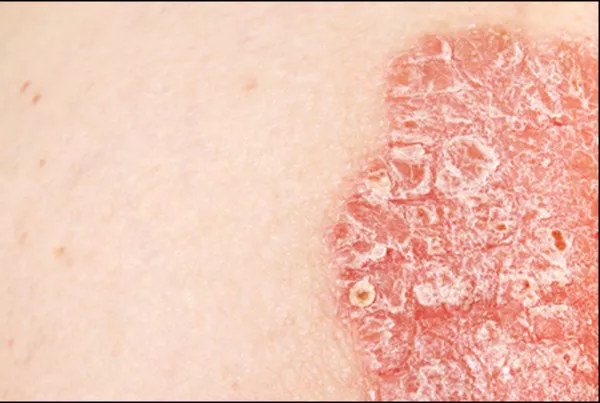
The U.S. Food and Drug Administration (FDA) has recently approved Zoryve (roflumilast) cream 0.15 percent for the treatment of mild to moderate atopic dermatitis in adults and children aged 6 and older. Atopic dermatitis, the most common form of eczema, can now be managed with this once-daily topical treatment, offering a rapid onset of action and fewer side effects compared to traditional therapies.
For decades, topical steroids and calcineurin inhibitors have been primary treatments for atopic dermatitis, but their long-term use and side effects have posed challenges for many patients. According to dermatologist Ray Kleinfelder, DO, these treatments are not always suitable due to safety concerns and discomfort associated with their application.
Zoryve represents a new approach as a phosphodiesterase 4 (PDE4) inhibitor. This mechanism targets inflammation in the skin preemptively, without relying on corticosteroids. Lawrence Eichenfeld, MD, a pediatric dermatologist involved in Zoryve’s clinical trials, highlights its innovative use of a well-tolerated moisturizer base to deliver potent anti-inflammatory benefits effectively.
Unlike topical steroids, which can be problematic on sensitive areas like the face, Zoryve offers versatility with minimal risk of adverse effects regardless of application site. Its efficacy was demonstrated in phase 3 clinical trials, where participants experienced significant symptom improvement, including reduced itching and clearer skin, as early as 24 hours after starting treatment.
In addition to its approval for atopic dermatitis, Zoryve was previously approved in higher concentrations for other dermatological conditions like plaque psoriasis and seborrheic dermatitis. Its safety profile was reinforced by the minimal incidence of side effects reported during trials, with common issues such as headache and nausea affecting less than 3 percent of participants.
Patients eager to explore Zoryve should consult their healthcare providers, as the medication is expected to be available for purchase by the end of July. While cost considerations may vary depending on insurance coverage, options like the Zoryve Direct Savings Card Program aim to alleviate financial burdens for eligible individuals.
Dr. Kleinfelder emphasizes the significance of Zoryve’s approval for pediatric use down to 6 years old, providing a much-needed alternative for young patients managing long-term atopic dermatitis. Despite potential cost challenges, initiatives like the Arcutis Cares patient assistance program aim to ensure accessibility for all patients in need of this innovative treatment option.
Related Topics: