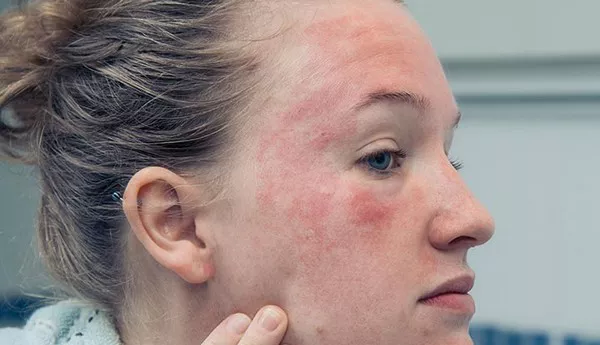
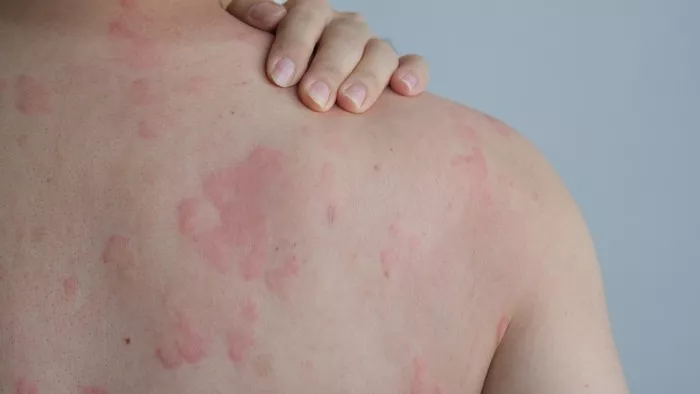
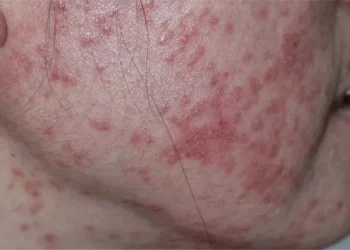
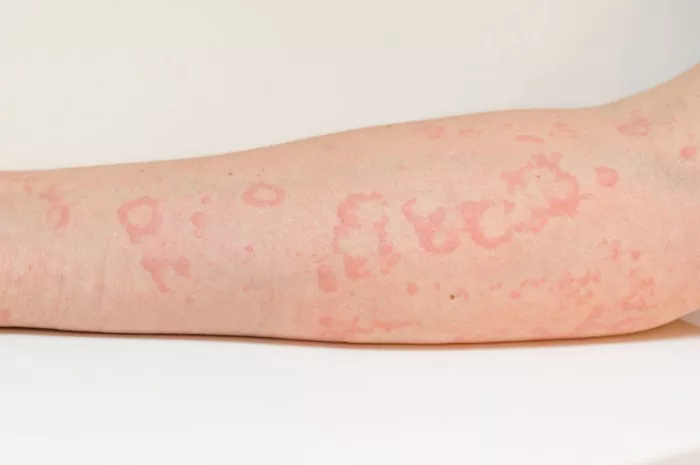
Patients with atopic dermatitis (AD) who use oral corticosteroids for more than 90 days may face a higher risk of adverse events, according to recent research published in JAMA Network Open. The study underscores the importance of ongoing monitoring for adverse effects in patients undergoing long-term oral corticosteroid treatment.
In the U.S., 7% to 11% of adults suffer from AD, also known as eczema, which is characterized by significant symptom management challenges, including visible skin lesions and persistent itching. While oral corticosteroids can alleviate these symptoms, limited research has been conducted on the potential adverse events associated with prolonged use in AD patients.
“International guidelines and expert opinions generally recommend that oral corticosteroids should be avoided or limited to short-term rescue therapy,” the study authors noted. “Despite their benefits—effectiveness in allergic diseases, short-term safety, and low cost—many patients with moderate to severe AD are treated with oral corticosteroids for extended periods, potentially leading to inappropriate or excessive use.”
Researchers from Kyungpook National University and Sungkyunkwan University conducted a nested case-control study to explore the link between long-term oral corticosteroid use and adverse events in adult AD patients. They utilized data from the Health Insurance Review and Assessment Service database of South Korea, spanning January 2012 to October 2021.
The study analyzed 164,809 patients with at least one prescription for oral corticosteroids between 2013 and 2020, matched with 328,303 control subjects. The patient cohort had a mean age of 39.4 years, with 56.9% being women and 43.1% men. Exclusion criteria included a diagnosis of immune-mediated inflammatory diseases within a year before cohort entry, a diagnosis of any of 11 specified outcomes, or being under 18 years old.
During the study period, 5,533 patients and 10,561 controls were exposed to oral corticosteroids for more than 30 days, while 684 patients and 1,153 controls used them for over 90 days. The researchers found no increased risk of adverse events with corticosteroid use exceeding 30 days, but a slight increase in risk for those using the medication beyond 90 days.
The most frequently observed adverse events in patients using oral corticosteroids for over 90 days included fractures, hyperlipidemia, myocardial infarction, and avascular necrosis.
The study acknowledged limitations such as potential discrepancies between recorded diagnoses and actual patient conditions, uncertain exposure measurements, exclusion of inhaled, topical, and eye drop corticosteroid formulations, and the inherent constraints of the case-control study design, which cannot entirely rule out reverse causality.
“In this large population-based case-control study, we identified a small increased risk of composite adverse outcomes associated with oral corticosteroid use exceeding 90 days in individuals with AD,” the authors concluded. “Further research is necessary to validate this potential risk, and healthcare professionals should carefully balance the benefits of oral corticosteroids against the observed small risk of adverse events, ensuring continuous monitoring during treatment.”
Related Topics: